B. Shrestha, J. M. Patt, A. Vertes
Analytical Chemistry, 2011, 83, 2947-2955
DOI: 10.1021/ac102958x | Impact factor (2011):
DOWNNLOAD | ONLINE
SUMMARY
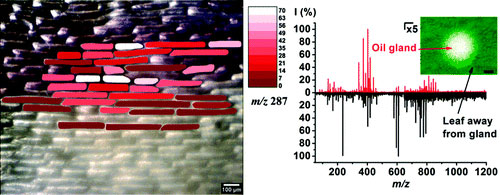
Molecular imaging by mass spectrometry (MS) is emerging as a tool to determine the distribution of proteins, lipids, and metabolites in tissues. The existing imaging methods, however, mostly rely on predefined rectangular grids for sampling that ignore the natural cellular organization of the tissue. Here we demonstrate that laser ablation electrospray ionization (LAESI) MS can be utilized for in situ cell-by-cell imaging of plant tissues. The cell-by-cell molecular image of the metabolite cyanidin, the ion responsible for purple pigmentation in onion (Allium cepa) epidermal cells, correlated well with the color of cells in the tissue. Chemical imaging using single-cells as voxels reflects the spatial distribution of biochemical differences within a tissue without the distortion stemming from sampling multiple cells within the laser focal spot. Microsampling by laser ablation also has the benefit of enabling the analysis of very small cell populations for biochemical heterogeneity. For example, with a 30 μm ablation spot we were able to analyze 3−4 achlorophyllous cells within an oil gland on a sour orange (Citrus aurantium) leaf. To explore cell-to-cell variations within and between tissues, multivariate statistical analysis on LAESI-MS data from epidermal cells of an A. cepa bulb and a C. aurantium leaf and from human buccal epithelial cell populations was performed using the method of orthogonal projections to latent structures discriminant analysis (OPLS-DA). The OPLS-DA analysis of mass spectra, containing over 300 peaks each, provided guidance in identifying a small number of metabolites most responsible for the variance between the cell populations. These metabolites can be viewed as promising candidates for biomarkers that, however, require further verification.